Chelate Test Page
This page will test the loading and presentation of a number of animated
GIF files created for an agricultural chelate page. Report any
difficulties to Phillip Barak
Note: the animated GIFs have proven to be too large for practical use
and have been largely replaced shortly with Chime and VRML representions.
See the Virtual
Museum of Minerals and Molecules.
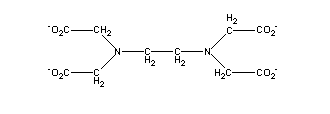 The
archtypal synthetic chelating agent is EDTA, commonly known as
ethylenediamine tetraacetic acid. The coordinating groups in EDTA are two
amine nitrogens and four carboxylic oxygens, which are capable of wrapping
around a central metal ion, such as Fe(III), and satisfying the octahedral
coordination requirements of Fe(III).
The
archtypal synthetic chelating agent is EDTA, commonly known as
ethylenediamine tetraacetic acid. The coordinating groups in EDTA are two
amine nitrogens and four carboxylic oxygens, which are capable of wrapping
around a central metal ion, such as Fe(III), and satisfying the octahedral
coordination requirements of Fe(III).
EDTA was the first synthetic chelate used in nutrient solutions for
hydroponics, although it was earlier noted that its effectiveness was
limited to pH 6 or lower. It is occasionally used in foliar application of
micronutrients. Soil applications are likewise limited to acidic and
slightly acidic soils, which makes its useful limited since most
micronutrient deficiencies that would call for chelate therapy appear in
calcareous soils, with pH greater than 7.5.
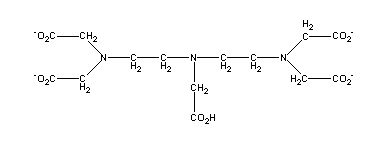 A
closely related synthetic chelate is DTPA, commonly known as
diethylenetriamine pentaacetic acid. The coordinating groups in DTPA are,
as in EDTA, two amine nitrogens and four carboxylic oxygens, which are
capable of wrapping around a central metal ion, such as Fe(III), and
satisfying the octahedral coordination requirements of Fe(III).
A
closely related synthetic chelate is DTPA, commonly known as
diethylenetriamine pentaacetic acid. The coordinating groups in DTPA are,
as in EDTA, two amine nitrogens and four carboxylic oxygens, which are
capable of wrapping around a central metal ion, such as Fe(III), and
satisfying the octahedral coordination requirements of Fe(III).
DTPA is widely used as an iron chelate in hydroponic solutions. It is
occasionally soil-applied as an iron or zinc source.
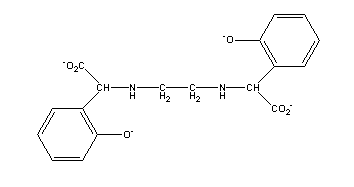 An
aromatic chemical relative of EDTA is EDDHA, commonly known as
ethylenediaminedi(o-hydroxyphenylacetic) acid, or EHPG
(N,N'-ethylenebis-2-(o-hydroxyphenyl) glycine). This molecule
offers two amine nitrogens, two carboxylic oxygens, and two phenolic
oxygens to satisfy octahedral coordination requirements. Because of the
strong bonds between phenolic groups and Fe(III), chelates of this type
are much stronger than purely carboxylic chelates. The phenol-Fe(III) bond
gives a red to purple color to the ferrated chelate.
An
aromatic chemical relative of EDTA is EDDHA, commonly known as
ethylenediaminedi(o-hydroxyphenylacetic) acid, or EHPG
(N,N'-ethylenebis-2-(o-hydroxyphenyl) glycine). This molecule
offers two amine nitrogens, two carboxylic oxygens, and two phenolic
oxygens to satisfy octahedral coordination requirements. Because of the
strong bonds between phenolic groups and Fe(III), chelates of this type
are much stronger than purely carboxylic chelates. The phenol-Fe(III) bond
gives a red to purple color to the ferrated chelate.
Examination of the chemical structure of EDDHA shows that
there are two asymmetrical carbon atoms around which are arranged
the acetic acid, phenol, and amino groups, along with an additional H to
make up the tetrahedral requirement of the carbon. The rules of
stereochemistry indicate that there are potentially 2n
stereoisomers, where n is the number of asymmetrical atoms, so
EDDHA could have four different stereoisomers. The rules of
stereochemistry assign a counting system around the asymmetrical atom,
based on various priorities, that yield either an R or S
designation for a particular arrangement around an asymmetric atom. When
EDDHA has one carbon with R and one S, it is identical to
EDDHA with one S and one R, so the total number of
stereoisomers is reduced by one. Stereoisomers that are superimposable on
their own mirror images are called meso compounds, and one of the
stereoisomers of EDDHA is therefore a meso stereoisomer.

|

|
| Fe(III)-meso-EDDHA: ball and stick
representation. [animated gif, 113 kb] |
Fe(III)-meso-EDDHA: space-filling
representation. [animated gif, 290 kb] |
|
When the meso-EDDHA coordinates with
Fe(III), one of the phenolic oxygens coordinates with Fe(III) in an
equatorial position and the other in a polar position, as shown in the
animations above. |

|

|
| Fe(III)-rac-EDDHA: ball and stick
representation. [animated gif, 113 kb] |
Fe(III)-rac-EDDHA: space-filling
representation. [animated gif, 290 kb] |
|
Two other stereoisomers are the R,R
and S,S isomers. These are enantiomers, stereoisomers
that are not superimposable on their mirror images. Equal amounts of
enantiomers are termed a racemic mixture, and lab synthesis of
molecules capable of stereoisomers typically produce racemic mixtures.
(Biological syntheses, on the other hand, are highly directed in terms
of specific stereochemistry.) The phenolic groups of the R,R
and S,S isomers are both arranged equatorially. |
The racemic and meso isomers of Fe-EDDHA can be
separated from each other using paper chromatography, ion chromatography,
and ion pair chromatography, as well as by careful crystallization. The
stability of Fe(III)-rac-EDDHA has been found to be 2.26 log units
higher than for the meso type, indicating a 500-fold difference in iron
chelating ability due to stereoisomerism! Whether this makes one
stereoisomer a better chelate for plant growth than the other is still
unresolved.
Although EDDHA was originally "advertised" as the phenolic
analog of EDTA, the truth is that EDDHA is an  -amino
acid, i.e., the amino and the carboxyl groups are bonded to the same
-amino
acid, i.e., the amino and the carboxyl groups are bonded to the same
 carbon (adjacent to the carboxyl C), while in EDTA, one acetic acid group
can be thought to be bonded directly to the amino group rather than the
carbon (adjacent to the carboxyl C), while in EDTA, one acetic acid group
can be thought to be bonded directly to the amino group rather than the
 carbon adjacent to the carboxyl C.
carbon adjacent to the carboxyl C.
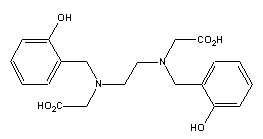 The
true phenolic analog to EDTA, then, is a different compound: HBED,
N,N'-bis(2-hydroxybenzyl)ethylenediamine-N,N'-diacetic acid. Examination
reveals that it does not have asymmetric carbons. However, chromatographic
evidence shows that Fe(III)-HBED may behave as if it has two asymmetric N
due to the very stable chelatation causing very slow equilibrium,
effectively producing two conformational isomers in equilibrium in a ~10:1
ratio.
The
true phenolic analog to EDTA, then, is a different compound: HBED,
N,N'-bis(2-hydroxybenzyl)ethylenediamine-N,N'-diacetic acid. Examination
reveals that it does not have asymmetric carbons. However, chromatographic
evidence shows that Fe(III)-HBED may behave as if it has two asymmetric N
due to the very stable chelatation causing very slow equilibrium,
effectively producing two conformational isomers in equilibrium in a ~10:1
ratio.
This page was last modified by
Phillip Barak, Univ. of
Wisconsin, on 14 Oct 1998. All rights reserved.
 The
archtypal synthetic chelating agent is EDTA, commonly known as
ethylenediamine tetraacetic acid. The coordinating groups in EDTA are two
amine nitrogens and four carboxylic oxygens, which are capable of wrapping
around a central metal ion, such as Fe(III), and satisfying the octahedral
coordination requirements of Fe(III).
The
archtypal synthetic chelating agent is EDTA, commonly known as
ethylenediamine tetraacetic acid. The coordinating groups in EDTA are two
amine nitrogens and four carboxylic oxygens, which are capable of wrapping
around a central metal ion, such as Fe(III), and satisfying the octahedral
coordination requirements of Fe(III).  A
closely related synthetic chelate is DTPA, commonly known as
diethylenetriamine pentaacetic acid. The coordinating groups in DTPA are,
as in EDTA, two amine nitrogens and four carboxylic oxygens, which are
capable of wrapping around a central metal ion, such as Fe(III), and
satisfying the octahedral coordination requirements of Fe(III).
A
closely related synthetic chelate is DTPA, commonly known as
diethylenetriamine pentaacetic acid. The coordinating groups in DTPA are,
as in EDTA, two amine nitrogens and four carboxylic oxygens, which are
capable of wrapping around a central metal ion, such as Fe(III), and
satisfying the octahedral coordination requirements of Fe(III). An
aromatic chemical relative of EDTA is EDDHA, commonly known as
ethylenediaminedi(o-hydroxyphenylacetic) acid, or EHPG
(N,N'-ethylenebis-2-(o-hydroxyphenyl) glycine). This molecule
offers two amine nitrogens, two carboxylic oxygens, and two phenolic
oxygens to satisfy octahedral coordination requirements. Because of the
strong bonds between phenolic groups and Fe(III), chelates of this type
are much stronger than purely carboxylic chelates. The phenol-Fe(III) bond
gives a red to purple color to the ferrated chelate.
An
aromatic chemical relative of EDTA is EDDHA, commonly known as
ethylenediaminedi(o-hydroxyphenylacetic) acid, or EHPG
(N,N'-ethylenebis-2-(o-hydroxyphenyl) glycine). This molecule
offers two amine nitrogens, two carboxylic oxygens, and two phenolic
oxygens to satisfy octahedral coordination requirements. Because of the
strong bonds between phenolic groups and Fe(III), chelates of this type
are much stronger than purely carboxylic chelates. The phenol-Fe(III) bond
gives a red to purple color to the ferrated chelate.



 The
true phenolic analog to EDTA, then, is a different compound: HBED,
N,N'-bis(2-hydroxybenzyl)ethylenediamine-N,N'-diacetic acid. Examination
reveals that it does not have asymmetric carbons. However, chromatographic
evidence shows that Fe(III)-HBED may behave as if it has two asymmetric N
due to the very stable chelatation causing very slow equilibrium,
effectively producing two conformational isomers in equilibrium in a ~10:1
ratio.
The
true phenolic analog to EDTA, then, is a different compound: HBED,
N,N'-bis(2-hydroxybenzyl)ethylenediamine-N,N'-diacetic acid. Examination
reveals that it does not have asymmetric carbons. However, chromatographic
evidence shows that Fe(III)-HBED may behave as if it has two asymmetric N
due to the very stable chelatation causing very slow equilibrium,
effectively producing two conformational isomers in equilibrium in a ~10:1
ratio.