Dear students: This page has been chemically-enhanced for your viewing pleasure. A browser plug-in (available for both Mac and PC and compatible with both Netscape and Internet Explorer) is required to view these interactive 3-D visualizations, available for free from MDL Informations Systems, Inc.. Download, install, restart your browser, and enjoy!
You've seen it before but there is no way that plant nutrient management can be considered without a thorough knowledge of the N cycle! Study Fig 5.1 in Tisdale et al. and recognize all of the following transformations:
Checklist for studying the N cycle, or you know that you know the N cycle when you know:
Interestingly, biological nitrogen fixation globally is 2/3 of the total annual N fixed per year and fixes more than twice as much N as humans do!
(Many of these losses are increased in magnitude by cultivation because of increased crop removal, seasonally bare soils, and increased oxidation/mineralization rates due to tillage.)
Be sure that you can identify these inputs and outputs as part of the N cycle!
Note that plants do not use nitrate and ammonium, directly but must reduce nitrate and assimilate them into organic compounds (with the minor exception of osmoregulation using nitrate above.) Reduction of nitrate takes place in both the root and the shoot. Particularly at high rates of nitrate supply or low photosynthetic activity, physiological limits to root reduction of nitrate will mean that increasing amounts of nitrate will be in the xylem flow, where it will be end up in shoots and ultimately be reduced in the leaves.
Nitrate reduced in the roots will be reduced first to nitrite by nitrate reductase and then to ammonium by nitrite reductase. Ammonium will undergo reaction with glutamate to form glutamine by the action of glutamine synthase. Glutamine may then undergo additional transformations before entering the xylem flow as reduced N, depending upon the plant species.
Nitrogen storage and transport compounds.
Plants do not do well with high concentrations of ammonia NH3 or
ammonium NH4+ floating around, so typically nitrate will
either be transported in the xylem fluid as nitrate or, if assimilated by
chemical reduction, as either amino acids or ureides (if a legume), for
example:
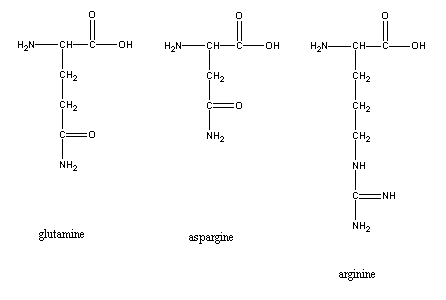
1) Glutamine
H2NC(=O)CH2CH2CH(NH2)COOH, 2N/5C
(=0.4)
2) Aspargine
H2NC(=O)CH2CH(NH2)COOH, 2N/4C (=0.5)
3)
Arginine
H2NC(=NH)NHCH2CH2CH2CH(NH2)COOH,
4N/6C (=0.67)

| Glutamine | Aspargine | Arginine |
| C | H | O | N |
| These interactive models are rotatable by dragging the cursor over the image, can be scaled in size by using the "Shift"+cursor, and can be displayed differently by choosing from a menu brought up by right-clicking (Mac: long clicking) on the image. | |||
These are not just any amino acids but those with particularly high N/C ratios. In this case, the C is the "packing material" and the plant does well to economize on the packaging since the C is photosynthetically-fixed at the cost of energy.
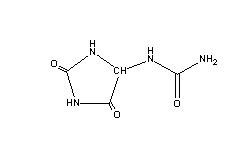
Ureides (such as allantoin, below containing 4N/4C = 1) are compounds produced by legumes as transport compounds that resemble polymerized urea, and require urease, an enzyme, to decompose them when they reach their destination.
 |
Next: Biological Nitrogen Fixation and Nitrogen fertilizers
Back to 326 HomePage
This page was last modified by Phillip Barak, Univ. of Wisconsin, on 24 Mar 1998. Chemically-enhanced on 26 Mar 1998. More about Chime at The Virtual Museum of Minerals and Molecules. All rights reserved.