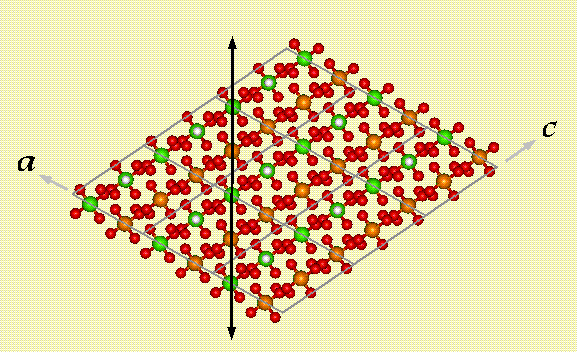
Dicalcium Phosphate Dihydrate (DCPD), also known as Brushite, is a hydrated calcium phosphate mineral with the composition CaHPO4·2H2O. The structure of DCPD is almost identical to that of gypsum with their respective unit cell dimesions very similar. The phosphate of DCPD is isomorphous with arsenate, which forms instead, the mineral Pharmacolite. The backbone of DCPD is made up of alternating calcium and phosphorus atoms which form chains via the sharing of oxygens (the four phosphate oxygens). These chains run perpendicular to the b axis in the plane formed by the a and c axes as shown in figure 1.

Figure 1. Calcium (green) and phosphorus (orange) chain
The phosphorus atoms have four fold coordination forming phosphate tetrahedra while the calcium atoms have eight fold coordination. Four of calcium's bonds are with these phosphate tetrahedra, forming the backbone chains, while two more of the calcium bonds are with oxygens from neighboring chains. These last bonds which link the chains together result in a zig-zag pattern or corrugated sheet. The last two calcium bonds are with the water molecules. These water molecules are what hold the sheets together.
Click on the buttons below to highlight specific features.
Calcium and phosphorus make up the majority of an animals mineral nutrient requirements (to fulfill both body tissue and milk needs), therefore DCPD is a common and widely used animal supplement. Dicalcium Phosphate Dihydrate is also of interest because it is the most soluble of the sparingly soluble calcium phosphate minerals and thus makes a good candidate for rock phosphate dissolution studies. The fate of DCPD in soils is rather transient. Typically, mineral phosphorus is added to soil in a water soluble form such as triple superphosphate or diammonium phosphate. As the phosphorus dissolves the high solution P concentration favors precipitation reactions. In neutral and calcareous soils, DCPD is one of the first reaction products. But the relatively soluble DCPD is unstable so after time (3 to 5 months) other more stable less soluble calcium phosphate minerals will form.
Beevers, C. A., Acta Cryst., (1958). 11,273