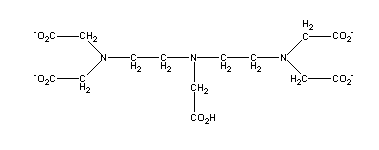
C14H23O10N3. Commonly known as DTPA (diethylenetriaminepentaacetic acid) or pentetic acid, this chelating agent is of the same general type as EDTA but forms stronger chelates with transition metals such as Fe and Zn.

This model shows the near-perfect coordination of the central Fe atom, with four carboxylate oxygens and three amino nitrogens as the apices of a pentagonal bipyramid. Fe-O bond lengths range between 1.94 and 2.05Å and the Fe-N bond lengths vary between 2.32 and 2.42Å. The symmetry of the central Fe atom with its nearest neighbors is a key factors in chelate stability.
In spacefilling mode, this model show that the central Fe atom is nearly completely concealed by coordinating oxygens and nitrogens. No coordination by additional water (H2O) or hydroxide (OH-) is permitted in this structure, a situation which adds to the stability of this chelate.
Identify the component groups:
| diethylenetriamine (H2NCH2CH2NHCH2CH2NH2) | |
| acetic acid [x5] (CH2COOH) |
This model is based upon the xyz coordinates of non-hydrogen atoms in crystals of Na2[FeDTPA]·2H2O determined from x-ray diffraction.
DC Finnen, AA Pinkerton, WR Dunham, RH Sands, and MO Funk, Jr. 1991. Structures and spectroscopic characteristics of iron(III) diethylenetriaminepentaacetic acid complexes. A non-heme iron(II) complex with relevance to the iron environment in lipoxygenases. Inorg. Chem. 30:3960-3964.