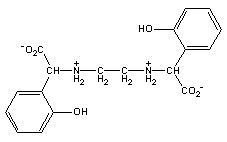
C18H20O6N2. This chelating agent is commonly known as EDDHA [ethylenediaminedi(o-hydroxyphenylacetic) acid] or EHPG [N,N'-ethylenebis-2-(o-hydroxyphenyl) glycine]. Because of the strong bonds between phenolic groups and Fe(III), chelates of this type are much stronger than those purely carboxylic chelating agents such as EDTA. The phenol-Fe(III) bond gives a red to purple color to the ferrated chelate.

This model shows the near-perfect octahedral coordination of the central Fe atom, with two carboxylate oxygens, two phenolate oxygens, and two amino nitrogens as the apices of the octahedron. The symmetry of the central Fe atom with its nearest neighbors is a key factors in chelate stability.
In spacefilling mode, this model show that the central Fe atom is nearly completely concealed by coordinating oxygens and nitrogens. No coordination by additional water (H2O) or hydroxide (OH-) is permitted in this structure, a situation which adds to the stability of this chelate.
Identify the component groups:
| ethylenediamine (H2NCH2CH2NH2) | |
| phenol [x2] (C6H6OH) | |
| acetic acid [x2] (CH2COOH) | |
| glycine [x2] (NH2CH2COOH) |
A tetrahedral carbon atom carrying four different groups constitutes a center of asymmetry and permits two arrangements in space. In EDDHA, there are two such asymmetric centers, and therefore 22 arrangements in space, or a total of 4 isomers. A mirror image of the FeEEDHA molecule above, also with phenolic groups in equatorial configuration, exists and a mixture of the two is a racemic mixture. The third and fourth configurations are actually identical to each other (due to the symmetry of the molecule itself) and are the meso configuration, the Fe chelate of which has a phenol group in both the equatorial and polar positions.
Fe-meso-EDDHA: