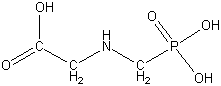
Common name: Glyphosate
IUPAC and Chemical Abstracts name: N-(phosphonomethyl)glycine
Molecular formula: C3H8NO5P;
Molecular weight:169.1

The acid dissociation constants for glyphosate are pKa1 0.8 (1st phosphonic), pKa2 2.3 (carboxylate), pKa3 6.0 (2nd phosphonic), and pKa4 11.0 (amine). As a result, this molecule has a tendency to dissociate one proton from the most acidic group and associate that proton with the most basic group, forming a dipolar molecule termed a zwitterion, as in the figure below.
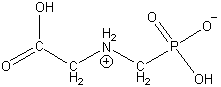
The double-bonded and the deprotonated oxygens of the phosphono group are in resonance with each other and have a slightly shorter P–O bond length (1.50 Å) than that of the P–OH bond (1.58 Å). Additional protons can be dissociated to form inorganic and organic salts of glyphosate.
Solubility: In water, 12 g/L @ 25 C; low solubility in organic solvents. Salts of gyphosate--ammonium, sodium, isopropylammonium, and trimesium-- are very soluble in water.
Usage: Nonselective systemic herbicide used to control many annual and perennial grasses and broadleaf weeds plus many tree and brush species. Applied to foliage and translocated throughout the plant, in which it prevents synthesis of essential aromatic amino acids needed for protein synthesis. Most commonly used in the isopropylammonium salt, marketed by Monsanto, the original patent-holder, as "Roundup". Additional basic producers and trademarked products are currently available.
Toxicology: Not an acute poison for mammals (e.g., oral LD50 for rats, 5600 mg/kg), not mutagenic, not carcinogenic, not teratogenic, not neurotoxic. Practically nontoic to fish, aquatic invertebrates, honeybees and earthworms.
Environmental Fate: Photodegrades in water under natural conditions, DT50 <=28 d. Strongly immobilized in soil and retained in top 15 cm, with DT50 in soil 3-174 d, depending on conditions. Major degradation product in soil, water, and plants is aminomethylphosponic acid, which is itself strongly adsorbed by soil and biodegradeable.
In recent years, glyphosate resistance has been genetically introduced into a number of agricultural plants, among them corn and soybeans, producing "Roundup-ready" varieties that can withstand post-emergence treatment of fields with glyphosate to kill weeds. Some controversy exists over both possible increased use of glyphosate in agriculture and the possibility of unintentional dissemination of genes for glyphosate resistance into weed species, making their control with glyphosate more difficult in the future.
Aside from agricultural uses, glyphosate is often used as the herbicide of choice for elimination of invasive plant species as part of environmental restoration efforts. It is also occasionally misused as a weed killer for lawn use (where the use of the broadleaf herbicide 2,4-D is indicated), killing both weeds and grass.
CAS RN: glyphosate, [1071-83-6]; ammonium-, [40465-66-5]; isopropylammonium-, [38641-94-0]
US Environmental Protection Agency (EPA). 1993.
Glyphosate:
Reregistration Eligibility Decision (RED) Fact Sheet
The Pesticide
Manual. 11th Ed. 1997. CDS Tomlin (ed.) The British Crop Protection Consortium.
Farm Chemicals Handbook '99. Willoughby, Ohio, Meister Pub. Co.
P.
Knuuttila and H. Knuuttila. 1979. The crystal and molecular structure of
N-(phosphonomethyl)glycine (glyphosate). Acta Chem. Scand. B
33:623-626.