The term smectite is used to describe a family of expansible 2:1 phyllosilicate minerals having permanent layer charge between 0.2 and 0.6 charges per half unit cell. Specific minerals included in the smectite family are montmorillonite, beidellite, saponite, nontronite, and several less common species.
Smectites are constructed of a single octahedral sheet sandwiched between two tetrahedral sheets, with the octahedral sheet sharing the apical oxygens of the tetrahedral sheet. The octahedral sheet may be either dioctahedral or trioctahedral. Layer charge arises from substitutions in either the octahedral sheet (typically from the substitution of low charge species such as Mg2+, Fe2+, or Mn2+ for Al3+ in dioctahedral species) or the tetrahedral sheet (where Al3+ or occasionally Fe3+ substitutes for Si4+), producing one negative charge for each such substitution.
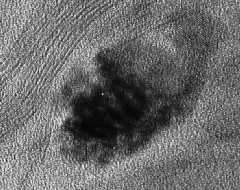
The interlayer (the space between the sheets) is hydrated and expansible; that is, the separation between individual smectite sheets varies depending on the interlayer cations present, the ionic strength of the surrounding solution, and other factors. The thumbnail image below is a TEM of a smectite embedded in a plastic resin after expansion of the interlayer with dodecalamine. Smectite layers are oriented both parallel to the C axis (the dark blob in the center of the image) and perpendicular to the image, as shown by the curving layers in the upper left part of the image.
 |
| Click on the image above for a larger image. |
Because the interlayer is hydrated, cations present within the interlayer may exchange with cations in the external solution providing charge balance is maintained.
Because the interlayer is expansible, smectites are referred to as "swelling clays". Smectite rich soils can undergo as much as a 30% volume change due to wetting and drying. These severe changes are responsible for the formation of the vast majority of soils in the Vertisol order, which form deep cracks upon drying. This expansion and contraction of the soil can cause extensive damage to building and their foundations, roads, and other structures.
Smectites commonly occur in soils and in source deposits. The majority of deposit-type smectites are classified as montmorillonites or occasionally beidellites. The properties and composition of soil smectites may be quite different from those of deposit-type smectites. Soil smectites commonly have higher charge than deposit smectites, contain more iron in the octahedral sheet, and often have a fairly even split between octahedral and tetrahedral charge.
The model shown has the composition of a soil smectite (Laird et al., 1991) derived from the Webster soil series in south central Minnesota. It's structure is based on the muscovite structure of Collins and Catlow (1992) with cation substitutions to accommodate differences in chemistry and changes in spacing between the 2:1 layers. The structure shown is based on a 14 Angstrom c-spacing.
The smectite sample shown is a high-charge (0.482 charges per formula unit) Fe-rich montmorillonite having 47% tetrahedral charge. The layer charge is derived from substitution of Mg2+ for Al3+ in the octahedral sheet and of Al3+ for Si4+ in the tetrahedral sheet. Charge balance is maintained by the presence of Na+ in the interlayer. The interlayer is hydrated (water molecules not shown) which allows the interlayer cations to exchange with cations in the external solution providing charge balance is maintained.
The following highlight buttons provide interactive functionality to the model. Just click on any of the buttons to highlight or illustrate the feature described.
| Show silicon (Si) atoms | |
| Show tetrahedral aluminum (Al) atoms | |
| Show octahedral aluminum atoms | |
| Show octahedral iron (Fe3+) atoms | |
| Show octahedral magnesium (Mg) atoms | |
| Show oxygen (O) atoms | |
| Show hydroxyls (OH) | |
| Show tetrahedral sheets | |
| Show octahedral sheets | |
| Show sites of negative charge | |
| Show charge-balancing cations (Na). Note that these are exchangeable cations. | |
| Show a single unit cell ** | |
| Show all atoms |
Smectite compositional data obtained from Laird et al. (1991). Crystallographic data for muscovite (Collins and Catlow, 1992) modified for the smectite structure and composition.
Collins, D.R., and C.A. Catlow. 1992. Computer simulation of structures and cohesive properties of micas. American Mineralogist 77:1172-1181.
Laird, D.A., P. Barak, E.A. Nater, and R.H. Dowdy. 1991. Chemistry of smectitic and illitic phases in interstratified soil smectite. Soil Science Society of America Journal 55:1499-1504.