Formula: C24H20BK (=K[BPh4])
![K[BPh4]](kph4b.gif)
The potassium ion, K+, is very soluble and very few precipitating agents are known. Tetraphenylborate was first synthesized in the 1950s and, in the sodium form, is very soluble in water. When K+ is introduced into solution, however, potassium tetraphenylborate is formed and precipitates. The solubility of K[BPh4] is very low in water (1.8x10-4 M), but is much higher in acetone and other organic solvents, including lipids.
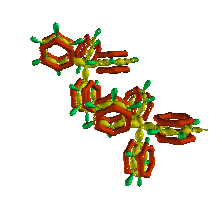
The crystal structure of KBPh4 shows that K+ is
coordinated with the p (pi) orbital of four benzene
rings from two adjacent KBPh4 molecules. In the nearby image,
K+ has been removed from the structure for purposes of clarity to
reveal the red "p-cage".
Tetraphenylborate also forms precipitates with ions of similar size and charge: NH4+, Rb+, Cs+, Ag+ and Tl+. Tetraphenylborate was originally proposed as an analytical reagent for determining K+ either gravimetrically or by titration; however, standard techniques of flame photometry, atomic absorption spectroscopy, and ICP-AES are all generally regarded as superior to this use. In laboratory settings, it has been used as an extractant for K in minerals, essentially weathering K-bearing minerals by reducing the K+ concentration in solution to nearly zero by precipitating K+. Tetraphenylborate has, from time to time, been proposed as an extractant for measuring quantities and fluxes of exchangeable and "fixed" K+ and NH4+ for evaluating soil fertility. It has also been proposed as a tool in the cleanup of radioactive spill sites in which radiocesium, Cs, is to be immobilized and recovered.
Flaschka, H., and A.J. Barnard, Jr. 1960. Tetraphenyl boron (TPB) as an analytical reagent. Adv. Anal. Chem. Instr. 1:1-117.
Hoffmann, K., and E. Weiss. 1974. J. Organometallic Chem. 67:221-228. [crystal structure].
Scott, A., R.R. Hunziker, and J.J. Hanway. 1960. Chemical extraction of potassium from soils and micaceous minerals with solutions containing sodium tetraphenylboron: I. Preliminary experiments. Soil Sci. Soc. Am. Proc. 24:191-194.