11 Nov. Agricultural Pollution: N & P Cycles, Pesticides
13 Nov. Agricultural Pollution: N & P Cycles, Pesticides
- 37. Pollution by Nutrient Losses
- Nitrogen losses through nitrate leaching amount to a few percent of the total losses from croplands each year. Nitrate leaches because: 1) soil colloids are negatively charged and, therefore, cannot retain negatively charged ions (anions such as nitrate) on their surfaces, and 2) nitrate is very soluble in water.
- Most nitrogen pollution of fresh waters results from humus in eroded soil and organic-rich wastes (e.g., manure and sewage sludge). Nitrogen loss by erosion is three times the nitrogen loss by nitrate leaching.
- Soil phosphorus is relatively insoluble and, therefore, leaching losses into groundwater is negligible. Excess phosphorus not used by plants remains in the soil, transformed from the very soluble compounds used as phosphorus fertilizers into insoluble soil minerals.
- Excessive phosphorus fertilization and land disposal of animal manure causes soil phosphorus build up in topsoil. Ammonia nitrogen in manure evaporates and soluble nitrate nitrogen leaches from manure exposed to rainfall. Consequently, the already high P/N ratio in manure increases overtime.
- 38. Eutrophication in Lakes and Streams
- Because certain algal species fix atmospheric nitrogen, most fresh water systems have adequate nitrogen fertility. Phosphorus minerals tend to be insoluble and, therefore, phosphorus is usually the nutrient that limits plant biomass in freshwater systems.
- Eutrophication occurs when the water becomes overly enriched with nutrients--usually phosphorus since it is the nutrient limiting biomass production.
- Phosphorus enrichment stimulates the growth (bloom) of fast-growing aquatic plants--typically algae. The biomass produced yields huge quantities of organic residue which stimulates a subsequent microbial bloom as the residue is degraded. The microbial bloom depletes the water of dissolved oxygen, killing aquatic fauna.
- Because phosphorus is relatively insoluble, eutrophication results from eroded sediments.
- Biological oxygen demand (BOD) is a technical term for wastes that are rich in organic carbon: manure, domestic sewage, leaves & grass clippings, food processing wastes (e.g., whey). The organic carbon in these wastes stimulate microbial blooms equivalent to those triggered by the die-back following an algal bloom, depleting water of dissolved oxygen and killing aquatic fauna.
- 39. Pollution Caused by Eroded Soil Sediments
- Sediments carry nutrients adsorbed to the surface of soil colloids--e.g., phosphorus.
- In certain circumstances, sediments may carry toxic elements adsorbed to the surface of soil colloids--e.g., selenium pollution in the San Joaquin Valley, California.
- Sediments carry pesticides adsorbed to soil humus.
- Sediment deposits fill reservoirs, lakes and clog streams.
- Suspended sediment reduce light penetration into lakes & streams and interfere with both aquatic plants and animals.
- 40. Pollution Caused by Pesticides
- Modern pesticides often mimic plant and insect hormones; causing death by disrupting metabolism, reproduction, or growth. Certain pesticides do not mimic hormones, but their mode of action interferes with essential life processes in the plant or insect.
- Ideal pesticides act rapidly, cause high mortality, kill specific organism without harming beneficial organisms, and do not persist in the environment. These goals are seldom achieved. Most pesticides are not specific--causing mortality in organisms that are not causing crop damage. Pesticides must be somewhat resistant to microbial or chemical degradation--otherwise they would not persist long enough to control the designated pest.
- First-generation pesticides were toxic inorganic compounds (calcium arsenate--CaAsO4, copper nitrate--CuNO3, copper sulfate--CuSO4, lead arsenate--PbAsO4) that will not degrade, required heavy application rates, and accumulate in soils. Second-generation pesticides were organic compounds that are highly-resistant to microbial degradation and, therefore, persistent in the environment. Modern insecticides are organic compounds that are biodegradable by soil microbes, less persistent in the environment and less likely to accumulate in species high in the food chain.
- Pesticides are organic compounds that adhere to soil humus, thereby reducing the likelihood of leaching and retaining the pesticide in the zone of biological activity where it can be degraded. Retention by soil humus increases the likelihood of transport by erosion.
16 Nov. Agricultural Pollution: N & P Cycles, Pesticides
18 Nov. Industrial Pollution: Acidity, Soil Colloids, Redox
20 Nov. Industrial Pollution: Acidity, Soil Colloids, Redox
- 41. Soil Colloids & Ion Exchange
- Ion exchange: the replacement of one adsorbed ion--on the surface of a soil colloid--by another ion dissolved in solution.
- Charge neutrality requires a balancing of charge during ion exchange.
- Soil colloids retain either cations and anions, depending on the surface charge of the soil colloid. The positive surface charge increases in acid soils, giving rise to anion exchange and the retention of anions. The negative surface charge increases in alkaline soils, giving rise to cation exchange and the retention of cations.
- Increased soil acidity increases the proportion of acid cations H+ held by soil colloids, which displace nutrient cations (magnesium--Mg2+ or calcium--Ca2+) and toxic cations (nickel--Ni2+ or lead--Pb2+) into soil water.
- Increased soil acidity increases the proportion of acid cations H+ held by soil colloids, which increases positive surface charge and removes nutrient anions (phosphate--PO43-) and toxic anions (chromate--CrO42-) from soil water.
- 42. Environmental Impact of Soil Acidification
- Plant growth and biological activity produce acidity through such natural processes as nutrient uptake by roots and respiration, but burning of fossil fuels and mining deposits containing sulfide minerals cause acidification through sulfide oxidation.
- Soil acidification reduces microbial activity, thereby lowering the effectiveness of biological remediation which depends on microbial activity to decompose organic wastes.
- Soil acidification reduces the retention of cations and increases both their solubility and, therefore, their biological availability and likelihood of transport by percolating water.
- 43. Cycling of Aerobic & Anaerobic Conditions
- Saturating soil with water, waterlogging, causes aerobic microbial communities to become dormant and anaerobic microbial communities to become active.
- Each type of soil organism uses a different type of compound to respire. Aerobic organisms use oxygen in the air: O2.
- Each anaerobic microbial community uses different compounds to respire in the absence of oxygen:
- Some anaerobic microbes (denitrifiying bacteria) use nitrate--NO3-
- Some anaerobic microbes use "oxidized" manganese minerals--MnO2
- Some anaerobic microbes use "oxidized" iron minerals--Fe2O3
- Some anaerobic microbes use sulfate--SO42-
- Some anaerobic microbes use carbonate--CO32---or carbon dioxide--CO2
- Reducing conditions can increase or decrease the biological availability of toxic elements. Toxic elements adhering to the surfaces of "oxidized" iron and manganese clays are released when reducing conditions cause these clays to dissolve. Some toxic elements—such as chromium, lead, and cadmium—become less soluble under reducing conditions.
23 Nov. Industrial Pollution: Acidity, Soil Colloids, Redox
25 Nov. Water Quality: Water Movement, Hydrology, Transport
27 Nov. Thanksgiving Recess
30 Nov. Water Quality: Water Movement, Hydrology, Transport
- 44. Water Flow Through Soil and Substrata
- Saturated flow is water flow caused by the pull of gravity. Saturated flow occurs at water contents between saturation and field capacity.
- Percolating water moving through the soil and substrata carries away dissolved compounds--nutrients and contaminants--in a process called leaching.
- The rate of saturated water flow depends upon permeability. Permeability controls the rate of water flow; it depends upon the size of the pores and their connectivity.
- Hydraulic conductivity K is a measure of permeability. It is the proportionality between the water flux and the force driving water flow.
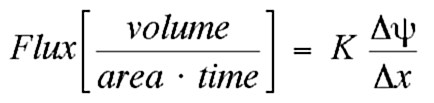
- Groundwater flows laterally and will transport contaminants in a plume that spreads and advances in the direction of groundwater flow.
- The rate of contaminant flow can equal the rate of groundwater flow if the contaminant does not adsorb (adhere) to the surface of soil colloids.
- If the contaminant adsorbs to soil colloids, it will migrate more slowly than the groundwater. The rate is often expressed as a retardation factor--a fraction of the actual groundwater flow rate.
- 45. Rates of Contaminant Decay
- All organic compounds are unstable in soil environments. A combination of chemical and biological processes transform organic substances--residue, humus or organic contaminants--into simpler compounds, ultimately carbon dioxide and water.
- Most degradation reactions occur at a constant rate, reducing the initial quantity of the substance by half during an interval called the half-life.
- The persistance of a contaminant, a measure of its resistance to chemical or biological degradation, is often given as its half-life. Easily degraded compounds may have a half-life of a few days or weeks while resistant compounds may have half-lives measured in years or decades.
- 46. Bioremediation
- Bioremediation is: The use of living organisms (primarily microorganisms) to degrade environmental pollutants or to prevent pollution through waste treatment.
- The "octanol-water" distribution coefficient KOW is the ratio between concentration of an organic contaminant in octanol and concentration of an organic contaminant in water. The partitioning of an organic contaminant between water and octanol is proportional to the bioaccumulation organic contaminants in the fatty tissue of animals.
- The soil distribution coefficient KD is the ratio between quantity of contaminant sorbed by whole soil or sediment and the contaminant concentration in water. The KD is a measure of the likelihood that a contaminant will adhere to soil colloids and resist leaching by percolating water.
- Soil humus has a high affinity for organic contaminants such as pesticides and industrial solvents. Scientists have found that the "octanol-water" distribution coefficient KOW for an organic contaminant is a good predictor of its soil distribution coefficient KD, which measures the likelihood soil humus will retard contaminant leaching.
- Contaminated sites vary in characteristics that affect pollutant migration and the ability of microbes to grow, including oxygen and nutrient concentrations and water availability.
- The most successful applications of bioremediation for site cleanup have been those that modify the environment to stimulate the activity of naturally occurring microbes. Factors that control the rate of biological activity (acidity, moisture content, aeration, nutrient availability, temperature) also control the rate of microbial degradation.